Hex Mayflies
Hexagenia limbata
The famous nocturnal Hex hatch of the Midwest (and a few other lucky locations) stirs to the surface mythically large brown trout that only touch streamers for the rest of the year.
Featured on the forum

It's only barely visible in one of my pictures, but I confirmed under the microscope that this one has a prosternal horn and the antennae are mid-way between the eyes and front of the head capsule.
I'm calling this one Pycnopsyche, but it's a bit perplexing. It seems to key definitively to at least Couplet 8 of the Key to Genera of Limnephilidae Larvae. That narrows it down to three genera, and the case seems wrong for the other two. The case looks right for Pycnopsyche, and it fits one of the key characteristics: "Abdominal sternum II without chloride epithelium and abdominal segment IX with only single seta on each side of dorsal sclerite." However, the characteristic "metanotal sa1 sclerites not fused, although often contiguous" does not seem to fit well. Those sclerites sure look fused to me, although I can make out a thin groove in the touching halves in the anterior half under the microscope. Perhaps this is a regional variation.
The only species of Pycnopsyche documented in Washington state is Pycnopsyche guttifera, and the colors and markings around the head of this specimen seem to match very well a specimen of that species from Massachusetts on Bugguide. So I am placing it in that species for now.
Whatever species this is, I photographed another specimen of seemingly the same species from the same spot a couple months later.
I'm calling this one Pycnopsyche, but it's a bit perplexing. It seems to key definitively to at least Couplet 8 of the Key to Genera of Limnephilidae Larvae. That narrows it down to three genera, and the case seems wrong for the other two. The case looks right for Pycnopsyche, and it fits one of the key characteristics: "Abdominal sternum II without chloride epithelium and abdominal segment IX with only single seta on each side of dorsal sclerite." However, the characteristic "metanotal sa1 sclerites not fused, although often contiguous" does not seem to fit well. Those sclerites sure look fused to me, although I can make out a thin groove in the touching halves in the anterior half under the microscope. Perhaps this is a regional variation.
The only species of Pycnopsyche documented in Washington state is Pycnopsyche guttifera, and the colors and markings around the head of this specimen seem to match very well a specimen of that species from Massachusetts on Bugguide. So I am placing it in that species for now.
Whatever species this is, I photographed another specimen of seemingly the same species from the same spot a couple months later.

Troutnut is a project started in 2003 by salmonid ecologist Jason "Troutnut" Neuswanger to help anglers and
fly tyers unabashedly embrace the entomological side of the sport. Learn more about Troutnut or
support the project for an enhanced experience here.
True Fly Family Chironomidae (Midges)

Some midges are large, up to hook size 14, but the majority are size 22 or smaller. The number of genera and species is hopelessly huge for angler entomologists to ever learn, and the identifing characteristics often require slide-mounting tiny parts under high-powered microscopes. Even the most Latin-minded fisherman must slip back to the basics--size and color--to describe his local midge hatches.
Hatching behavior
Midges rise to the surface as pupae and struggle slowly through the surface film while the pupa's body dangles vertically below. This is the most common stage for trout to take, though the adults may be useful at times too.Midge pupae account for much of the mystifying midsummer spring creek action on evenings when no bugs seem to be in the air or on the water, yet trout are rising everywhere and ignoring one's flies. Recognizing a midge hatch is far from a guarantee of fish, however. Suitable imitation is not easy.
Larva & pupa biology
Substrate: Usually silt or detritus
Environmental tolerance: Best in clear water
Chironomidae Fly Fishing Tips
Despite the tiny size of midges, trout can be very selective to their size and color. Remember that a difference of a single hook size in the tiny sizes is a very large percentage difference and very noticeable by the trout. Netting some of the real insects before choosing a fly is surely a good idea, but it's easier said than done.Specimens of Midges:
1 Female Adult

This midge and several like it, including a male I also photographed, hatched from larvae which were living in some fine mud I'm using as substrate in my bug-rearing aquarium. This one flew away before I could photograph it on the ruler, but it would have measured slightly smaller than the male.
5 Adults
1 Male Adult

This midge and several like it, including a female I also photographed, hatched from larvae which were living in some fine mud I'm using as substrate in my bug-rearing aquarium.
3 Pupae
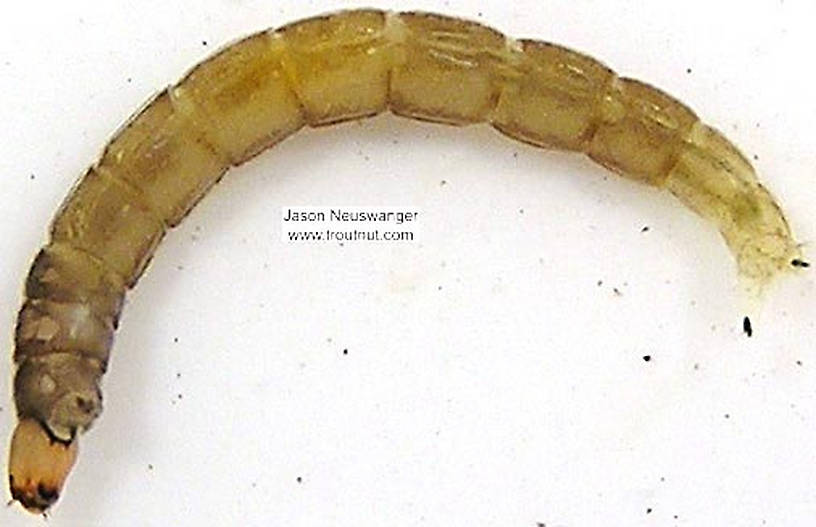
15 Larvae
3 Streamside Pictures of Midges:
4 Underwater Pictures of Midges:
Discussions of Chironomidae
getting midges down
9 replies
Posted by CaseyP on Dec 8, 2007
Last reply on Jan 17, 2018 by Ummm
says here midge pupae rise in the water and struggle in the film to become adults. my imitations are so small that they don't seem to go down very far in order to rise--ike a wet fly might at the end of the swing. any ideas? or am i fishing them wrong?
Clusters of midges and the Griffith's Gnat
35 replies
Posted by Troutnut on Apr 10, 2007
Last reply on May 22, 2015 by Taxon
This is a spin-off from a tangent in another topic which seems worthy of its own thread.
I wrote about the midge I photographed:
Gonzo replied:
Those are the explanations I've heard, too, but I'm skeptical of both. I'm not doubting the effectiveness of the Griffith's Gnat; I just think people have traditionally stretched the bounds of credibility when trying to explain a fly's success in imitative terms, and this is one of the more prominent examples.
Has anybody here seen a cluster of midges on the water? I haven't. I have seen midges thickly grouped on rocks next to the water, and I don't doubt that they occasionally fall off of there, and probably sometimes two are three are clinging to each other. I've also seen early-season stoneflies balled up with each other in an opaque little (presumably mating-related) clump on a midstream boulder, and I wouldn't be surprised if some midge species do something like that too. But trout don't see balls of midges floating around very often on any stream I've ever observed. Has anyone experienced that?
In either case, I can't see something so opaque as a Griffith's Gnat effectively imitating what would surely be a loose ball of gangly entangled midges. You would have to roll them around in your fingers for a while to goo them together so solidly.
I just can't see the herl and hackle imitating, or even suggesting, a loosening pupal shuck. Shucks don't get that loose. They trail behind length-wise; they don't balloon to the sides. And they aren't pointy. Of course I'm sure Schwiebert knows all that and was just trying to add an idea to the mix of explanations, but I do find that one as far-fetched as the others.
Here's another far-fetched guess: maybe the hackle and refractive trickery in the surface film reduce the perceived thickness of the fly and it passes for a single midge pretty well. This could be tested in an aquarium but it's late and I'm feeling lazy.
I think it's more likely that when trout take a Griffith's Gnat they're only looking for (at most) the right general size and color. It's not as fun, but sometimes things are really that simple.
I wrote about the midge I photographed:
I'm not sure how a griffith's gnat is supposed to imitate such a thing
Gonzo replied:
Schwiebert's theory was that when it was awash in the film, the herl and halo of hackle suggested the loosening pupal shuck around the dark body of the emerging midge. Others have speculated that it imitates a cluster of midges. Both theories are reasonable, I suppose, depending on the size of the Griffith's Gnat that is effective relative to the size of the actual midge. Like many anglers, I just know that it does work. :)
Those are the explanations I've heard, too, but I'm skeptical of both. I'm not doubting the effectiveness of the Griffith's Gnat; I just think people have traditionally stretched the bounds of credibility when trying to explain a fly's success in imitative terms, and this is one of the more prominent examples.
Has anybody here seen a cluster of midges on the water? I haven't. I have seen midges thickly grouped on rocks next to the water, and I don't doubt that they occasionally fall off of there, and probably sometimes two are three are clinging to each other. I've also seen early-season stoneflies balled up with each other in an opaque little (presumably mating-related) clump on a midstream boulder, and I wouldn't be surprised if some midge species do something like that too. But trout don't see balls of midges floating around very often on any stream I've ever observed. Has anyone experienced that?
In either case, I can't see something so opaque as a Griffith's Gnat effectively imitating what would surely be a loose ball of gangly entangled midges. You would have to roll them around in your fingers for a while to goo them together so solidly.
I just can't see the herl and hackle imitating, or even suggesting, a loosening pupal shuck. Shucks don't get that loose. They trail behind length-wise; they don't balloon to the sides. And they aren't pointy. Of course I'm sure Schwiebert knows all that and was just trying to add an idea to the mix of explanations, but I do find that one as far-fetched as the others.
Here's another far-fetched guess: maybe the hackle and refractive trickery in the surface film reduce the perceived thickness of the fly and it passes for a single midge pretty well. This could be tested in an aquarium but it's late and I'm feeling lazy.
I think it's more likely that when trout take a Griffith's Gnat they're only looking for (at most) the right general size and color. It's not as fun, but sometimes things are really that simple.
Midges
10 replies
Posted by Goose on Oct 24, 2006
Last reply on Jan 14, 2015 by Martinlf
Hi Jason & Gonzo! I did well over the weekend by fishing some midge pupa imitations as nymphs and in the film. Where does one find midge larva to inspect for pattern imitation? I fished the pupas and larvas on 5x tippet and it seemed to work well. Do midges live in the silt or on the bottom of rocks, etc.? I have the book by Ed Koch and ?, the other name escapes me for the moment, but it doesn't exactly say where they are found. Midges are about the game in in town during the winter months so I thought I'd learn a little about them. Thanks
Life cycles and hatches
3 replies
Posted by Leahdanger on Mar 7, 2014
Last reply on Mar 9, 2014 by Entoman
Hi,
I am trying to identify the life cycle of a few aquatic insects in eastern British Columbia. Specifically, I want to know if the species I collected in the fall (Aug-Oct) are a different hatch from the species I collected in the spring (April-May). Does anybody know when the following species lay eggs, die, and hatch: amphipods (Hyalella aztecha), chironomids, leeches, mayflies (Leptophlebiidae paraleptophlebia, Baetida sp.), and caddis flies (Oxyethira sp., Hydropsychidae arctopsyche)? For my purposes, knowing the general life cycle of amphipods and chironomids would be great.
Thanks much for your help!
Leah
I am trying to identify the life cycle of a few aquatic insects in eastern British Columbia. Specifically, I want to know if the species I collected in the fall (Aug-Oct) are a different hatch from the species I collected in the spring (April-May). Does anybody know when the following species lay eggs, die, and hatch: amphipods (Hyalella aztecha), chironomids, leeches, mayflies (Leptophlebiidae paraleptophlebia, Baetida sp.), and caddis flies (Oxyethira sp., Hydropsychidae arctopsyche)? For my purposes, knowing the general life cycle of amphipods and chironomids would be great.
Thanks much for your help!
Leah
Midge Video
5 replies
Posted by JAD on Mar 4, 2010
Last reply on Mar 4, 2010 by Martinlf
I thing some members will like this video on midges.
http://www.midcurrent.com/video/clips/cutter_midge.aspx
Best
JAD
http://www.midcurrent.com/video/clips/cutter_midge.aspx
Best
JAD
Start a Discussion of Chironomidae
References
- Schwiebert, Ernest G. 1955. Matching the Hatch. MacMillan Publishing Company.
- Swisher, Doug and Carl Richards. 2000. Selective Trout. The Lyons Press.
True Fly Family Chironomidae (Midges)
Taxonomy
Genus in Chironomidae
Chironomus
0
0
Prodiamesa
1
1
Rheotanytarsus
1
6
Stenochironomus
1
6
Stictochironomus
1
11
194 genera (Abiskomyia, Ablabesmyia, Acamptocladius, Acricotopus, Alotanypus, Antillocladius, Apedilum, Apometriocnemus, Apsectrotanypus, Arctodiamesa, Arctopelopia, Asheum, Axarus, Baeoctenus, Beardius, Beckidia, Bethbilbeckia, Boreochlus, Boreoheptagyia, Brillia, Brundiniella, Bryophaenocladius, Camptocladius, Cantopelopia, Cardiocladius, Chaetocladius, Chasmatonotus, Chernovskiia, Cladopelma, Cladotanytarsus, Clinotanypus, Clunio, Coelotanypus, Compteromesa, Compterosmittia, Conchapelopia, Constempellina, Corynocera, Corynoneura, Cricotopus, Cryptochironomus, Cryptotendipes, Cyphomella, Demeijerea, Demicryptochironomus, Denopelopia, Derotanypus, Diamesa, Dicrotendipes, Diplocladius, Diplosmittia, Djalmabatista, Doithrix, Doncricotopus, Einfeldia, Endochironomus, Endotribelos, Epoicladius, Eretmoptera, Eukiefferiella, Euryhapsis, Fittkauimyia, Georthocladius, Gillotia, Glyptotendipes, Goeldichironomus, Guttipelopia, Gymnometriocnemus, Halocladius, Harnischia, Hayesomyia, Heleniella, Helopelopia, Heterotanytarsus, Heterotrissocladius, Hudsonimyia, Hydrobaenus, Hyporhygma, Kiefferulus, Kloosia, Krenopelopia, Krenosmittia, Labrundinia, Lappodiamesa, Larsia, Lasiodiamesa, Lauterborniella, Limnophyes, Lipurometriocnemus, Lopescladius, Macropelopia, Meropelopia, Mesocricotopus, Mesosmittia, Metriocnemus, Microchironomus, Micropsectra, Microtendipes, Monodiamesa, Monopelopia, NAnocladius, Natarsia, Nilotanypus, Nilothauma, Nimbocera, Odontomesa, Oliveridia, Omisus, Oreadomyia, Orthocladius, PSeudokiefferiella, Pagastia, Pagastiella, Paraboreochlus, Parachaetocladius, Parachironomus, Paracladius, Paracladopelma, Paracricotopus, Parakiefferiella, Paralauterborniella, Paralimnophyes, Paramerina, Parametriocnemus, Paraphaenocladius, Parasmittia, Paratanytarsus, Paratendipes, Paratrichocladius, Paratrissocladius, Parochlus, Pentaneura, Phaenopsectra, Platysmittia, Plhudsonia, Polypedilum, Potthastia, Procladius, Protanypus, Psectrocladius, Psectrotanypus, Pseudochironomus, Pseudodiamesa, Pseudorthocladius, Pseudosmittia, Psilometriocnemus, Radotanypus, Rheocricotopus, Rheomyia, Rheopelopia, Robackia, Saetheria, Saetheriella, Sergentia, Skutzia, Smittia, Stackelbergina, Stelechomyia, Stempellina, Stempellinella, Stilocladius, Sublettea, Sublettiella, Symbiocladius, Sympotthastia, Synendotendipes, Synorthocladius, Tanypus, Tanytarsus, Tavastia, Telmatogeton, Telopelopia, Tethymyia, Thalassosmittia, Thanossomya, Thienemannia, Thienemanniella, Thienemannimyia, Tokunagaia, Tribelos, Trichochilus, Trichocladius, Trichotanypus, Trissopelopia, Tvetenia, Unniella, Vivacricotopus, Xenochironomus, Xenopelopia, Xestochironomus, Xylotopus, Zalutschia, Zavreliella, and Zavrelimyia) aren't included.