
Blue-winged Olives
Baetis
Tiny Baetis mayflies are perhaps the most commonly encountered and imitated by anglers on all American trout streams due to their great abundance, widespread distribution, and trout-friendly emergence habits.
Featured on the forum

This is the first of it's family I've seen, collected from a tiny, fishless stream in the Cascades. The three species of this genus all live in the Northwest and are predators that primarily eat stonefly nymphs Merritt R.W., Cummins, K.W., and Berg, M.B. (2019).

Troutnut is a project started in 2003 by salmonid ecologist Jason "Troutnut" Neuswanger to help anglers and
fly tyers unabashedly embrace the entomological side of the sport. Learn more about Troutnut or
support the project for an enhanced experience here.
Troutnut on Nov 14, 2020November 14th, 2020, 3:29 pm EST
I recently got a new microscope (AmScope 3.5X-180X Simul-Focal Trinocular Stereo Zoom Microscope and 18MP USB3 Camera) thanks to some generous family members on a major birthday. (I'm still not sure how 40 happened; I was 39 and then not so much.) I've spent some time over the past week catching up on bug IDs, specifically all the specimens I have preserved in ethanol from this past summer and part of the previous summer. I've also got some new dissecting tools: a minitool set, micro dissecting kit, and a petri dish with a self-healing silicone rubber bottom for pinning bugs into position. Between these new toys and a bit of help from taxonomic experts on a few specimens (thanks Dave Ruiter and Luke Jacobus), I've narrowed down the IDs on most of these recent finds. Browsing the front page of the site and clicking through the first several pages will take you to most of those bugs.
It is fantastic to be able to take pictures of the relevant features as I work through the keys and easily upload them to go along with my DSLR pictures from each specimen. I'm sure I'll get better at capturing and processing pictures from the scope to take clear pictures, but in the meantime here are a few of the pictures from a few of the specimens I recently identified with the scope:
Isoperla fusca

The mesobasisternum on stoneflies always used to give me a hard time despite its prominent place in the family keys. Not anymore.

Baetis tricauatus -- I'm not 100 % sure on this ID, but the microscope got me close.

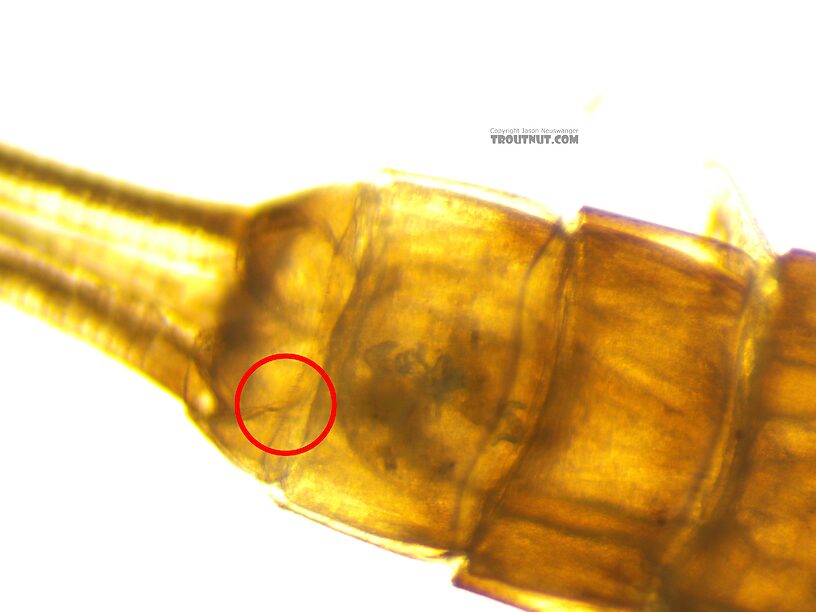
Some ridiculously tiny setae on the abdomen were revealed by the new scope and important to the key:
Doroneuria baumanni

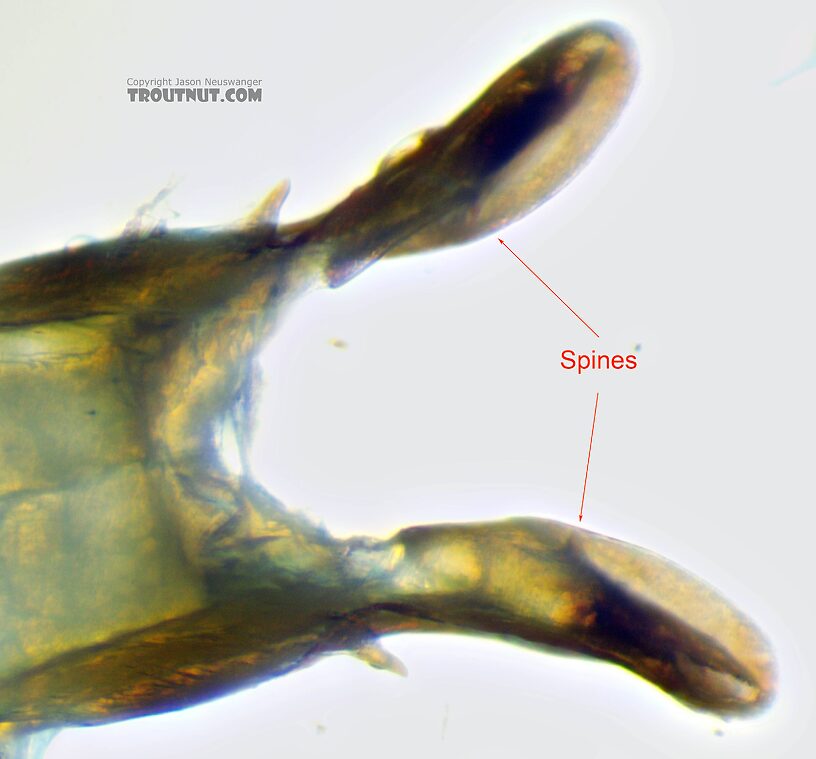
I'm finally starting to be able to get decent images of the reproductive anatomy of most of my specimens, which is an enormous help in keying male adults to species:

Speaking of which, Rhithrogena hageni

The new scope made all the difference in being able to confidently ID this species:
It is fantastic to be able to take pictures of the relevant features as I work through the keys and easily upload them to go along with my DSLR pictures from each specimen. I'm sure I'll get better at capturing and processing pictures from the scope to take clear pictures, but in the meantime here are a few of the pictures from a few of the specimens I recently identified with the scope:
Isoperla fusca

The mesobasisternum on stoneflies always used to give me a hard time despite its prominent place in the family keys. Not anymore.

Baetis tricauatus -- I'm not 100 % sure on this ID, but the microscope got me close.

Some ridiculously tiny setae on the abdomen were revealed by the new scope and important to the key:

Doroneuria baumanni

I'm finally starting to be able to get decent images of the reproductive anatomy of most of my specimens, which is an enormous help in keying male adults to species:

Speaking of which, Rhithrogena hageni

The new scope made all the difference in being able to confidently ID this species:

Jason Neuswanger, Ph.D.
Troutnut and salmonid ecologist
Troutnut and salmonid ecologist
Partsman on Nov 14, 2020November 14th, 2020, 11:18 pm EST
Amazing!
Taxon on Nov 15, 2020November 15th, 2020, 11:33 am EST
Hi Jason-
I (belatedly) wish you a happy 40th birthday.
I (belatedly) wish you a happy 40th birthday.
Red_green_h on Nov 16, 2020November 16th, 2020, 6:49 am EST
40 isn't the end of the world, heck I didn't start fly fishing until I turned 40. I feel like I'm 20 years younger out there now.
I've really come to appreciate the aquatic insect encyclopedia on this forum as I am attempting to identify the different species in the places I go to fish in New Mexico. There isn't a lot of info out there regarding species in this area. About the only documentation I have found save for an article here and there come from issues of the Great Basin Naturalist back in 1990s. And some of those findings are from late 1890s!!!
Being my eyesight is so bad it has really become a bonding father/son time being my 12 year old is a junior entomologist as he has really been my eyes in looking for and trying to identify different species. He always has been a bug guy. I have picture after picture with praying mantis's crawling all over him. So combining his love for bugs and his growing love for fly fishing is like a dream come true at least for me.
Thank you for your diligence in procuring this forum, and a thanks to you too Roger for your website (it has proven very helpful). I for one, as I'm sure many others, have found it indispensable. Every time I come on this site I am thankful for being able to glean from everyone who contributes.
I've really come to appreciate the aquatic insect encyclopedia on this forum as I am attempting to identify the different species in the places I go to fish in New Mexico. There isn't a lot of info out there regarding species in this area. About the only documentation I have found save for an article here and there come from issues of the Great Basin Naturalist back in 1990s. And some of those findings are from late 1890s!!!
Being my eyesight is so bad it has really become a bonding father/son time being my 12 year old is a junior entomologist as he has really been my eyes in looking for and trying to identify different species. He always has been a bug guy. I have picture after picture with praying mantis's crawling all over him. So combining his love for bugs and his growing love for fly fishing is like a dream come true at least for me.
Thank you for your diligence in procuring this forum, and a thanks to you too Roger for your website (it has proven very helpful). I for one, as I'm sure many others, have found it indispensable. Every time I come on this site I am thankful for being able to glean from everyone who contributes.
Wiflyfisher on Nov 17, 2020November 17th, 2020, 11:08 am EST
Nice!
Ethanol? I have been using 90% isopropyl. Is ethanol better?
Ethanol? I have been using 90% isopropyl. Is ethanol better?
John S.
https://WiFlyFisher.com
https://WiFlyFisher.com
Millcreek on Nov 17, 2020November 17th, 2020, 1:34 pm EST
If you're looking for a determination for DNA then ethanol is best, otherwise isopropyl is fine for preservation and cheaper.
"If we knew what it was we were doing, it would not be called research, would it?"
-Albert Einstein
-Albert Einstein
Wbranch on Nov 17, 2020November 17th, 2020, 11:44 pm EST
Happy belated birthday Jason! At 40 I was still single and fishing as often as possible. I was finally becoming a very good fly tier and catching more and more big trout. With good health you can expect to fish another forty years.
Catskill fly fisher for fifty-five years.
Troutnut on Nov 18, 2020November 18th, 2020, 6:38 am EST
The reasons I've been taught for using ethanol vs isopropyl in professional collections (mainly drift net and fish diet samples) is that ethanol preserves more flexibility in the specimens, whereas isopropyl can make them more brittle and likely to break off legs, tails, etc. That's less of an issue if you're preserving specimens individually or just a few at a time for personal reference, but it comes into play when we're bagging 300 bugs with a bunch of debris in a drift net sample and having a lab sort through them 6 months later. I just use it for my Troutnut collections out of habit.
Thanks for the birthday wishes!
Thanks for the birthday wishes!
Jason Neuswanger, Ph.D.
Troutnut and salmonid ecologist
Troutnut and salmonid ecologist
Jmd123 on Nov 19, 2020November 19th, 2020, 12:30 am EST
The standard for long-term preservation is 70% ethanol, which maintains flexibility in specimens. Both 95% ethanol and isopropyl will make them brittle as per Jason's comments. I am currently working my way through thousands of inverts collected on the job (field trip to Indiana) and I'm using 70% ethanol.
(Troutnut story coming on this trip!)
Jonathon
(Troutnut story coming on this trip!)
Jonathon
No matter how big the one you just caught is, there's always a bigger one out there somewhere...
Creno on Dec 5, 2020December 5th, 2020, 4:35 am EST
The choice of preservative should also consider the user, especially kids. If you are working on lots of material you should avoid most preservatives other than ETOH. Breathing, like drinking, isopropyl is not healthy. ETOH is becominmg more readily available, and cheaper, as local laws change on its sale as a result of craft distillaries. Most of the cost is taxes, not the ETOH. You can also make your own now in many states.
Quick Reply
Related Discussions
Topic
Replies
Last Reply
2
Sep 20, 2020
by Creno
by Creno
3
Feb 12, 2009
by Creno
by Creno
Re: DIY Photo-Microscope with a smart cell phone, iPod Touch, iPad (aka Smart-D-Scope)
In the Photography Board by LowBudget
In the Photography Board by LowBudget
1
Apr 1, 2014
by Entoman
by Entoman
6
Apr 14, 2013
by Troutnut
by Troutnut
Re: Tiny Black Caddis hatch, late fall - Nov 26 to be exact_ Spring Creek Southern MO
In the Caddisfly Family Hydroptilidae by Dai_sca
In the Caddisfly Family Hydroptilidae by Dai_sca
21
Dec 10, 2019
by Pdcox
by Pdcox








